Demystifying Diabetes
 Diagnosis of diabetes throws more questions than answers. To lessen the misery and mystery of diabetes, we need to clarify misconceptions prevalent about the disease…
Diagnosis of diabetes throws more questions than answers. To lessen the misery and mystery of diabetes, we need to clarify misconceptions prevalent about the disease…
By Dr Ramesh G Goyal
Diabetes is a common problem in older adults. Approximately 20% of individuals over 65 years of age have diabetes mellitus, and almost half of these individuals have not been diagnosed.
Unlike younger people with type-II diabetes, who are often overweight, obesity is not that common among older diabetes patients.
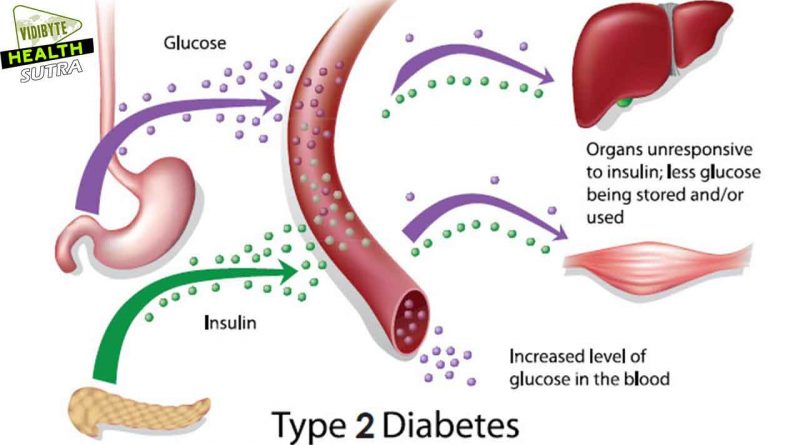
However, there are widespread misconceptions about possible consequences of uncontrolled hyperglycemia, a condition in which an excessive amount of glucose circulates in the blood plasma. In nursing homes, the problem of being underweight is as common as that of being overweight. Thus, nutritional management should focus on weight gain for underweight elderly patients as much as it is focused on weight loss for obese patients.
In addition to diet and exercise, pharmacological therapy is often required for optimizing blood glucose control.
Target blood glucose ranges should be individualized. In frail patients, fasting plasma glucose levels should range from 100 to 140 mg/dl, and postprandial values should be <200 mg/dl.
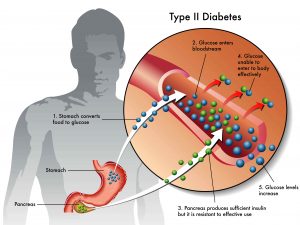
 The discovery of several classes of oral antidiabetic agents has increased the prospects of achieving better control of hyperglycemia with reduced risk of severe adverse events.
The discovery of several classes of oral antidiabetic agents has increased the prospects of achieving better control of hyperglycemia with reduced risk of severe adverse events.
Some of these agents, such as acarbose, miglitol, metformin, and troglitazone, do not cause hypoglycemia when used as monotherapy. As such, they are safer agents.
On the other hand, the low cost of some sulfonylurea agents and a once- or twice-daily administration schedule make them an attractive option.
Metformin appears to be especially useful in obese insulin-resistant patients. The available data on safety and efficacy of troglitazone in the elderly is insufficient.
The use of a combination of two or three oral antidiabetic agents to delay the need for insulin therapy is now possible. The long-term effects of this approach are not known, and the cost of polypharmacy is of concern.
Prior to 1979, at least six different sets of criteria diagnosed diabetes. In 1979, the National Diabetes Data Group (NDDG) resolved this issue by establishing one set of criteria.
They selected these criteria based on glucose concentrations that allegedly predicted the development of diabetic retinopathy, a specific microvascular complication of diabetes. Three prospective studies were available to the NDDG on which to base their decision. A total of 1,213 patients were followed for 3 to 8 years after oral glucose tolerance tests (OGTTs), 77 of whom developed retinopathy.
There was no further evaluation of their glycemic status after the original OGTT, although it was very likely that the 77 people who developed retinopathy in the studies used by the NDDG to establish the diagnostic criteria had increasing glycemia in the years between the test and the identification of retinopathy. However, on the basis of these 77 individuals, the NDDG selected a fasting plasma glucose (FPG) concentration of ≥140 mg/dL or a 2-h value after 75 gram oral glucose of ≥200 mg/dL to diagnose diabetes. Thus, the “gold standard” 2-h value on an OGTT for diagnosing diabetes rests on fewer than 100 individuals whose glycemic status was unknown for years prior to the development of retinopathy. A description of the three studies used for their decision is available.
In the mid-1990s, the American Diabetes Association (ADA) convened an Expert Committee to reexamine the diagnosis of diabetes in light of any new information available since the NDDG report. An overriding goal of the committee was to make the FPG concentration and the 2-h glucose concentration on the OGTT equivalent for the diagnosis of diabetes, that is, if one criterion was met, the other would likely be met as well).
 With the NDDG criteria, ∼95% of patients whose FPG concentrations were 140 mg/dL had 2-h glucose concentrations ≥200 mg/dL on the OGTT (17), but only one-quarter to one-half of patients with 2-h values on the OGTT ≥200 mg/dL had FPG concentrations ≥140 mg/dL (17–19). The Expert Committee decided to retain the 2-h glucose concentration of ≥200 mg/dL as a diagnostic criterion because changing it “would be very disruptive” considering the large number of epidemiological studies using that value to define diabetes.
With the NDDG criteria, ∼95% of patients whose FPG concentrations were 140 mg/dL had 2-h glucose concentrations ≥200 mg/dL on the OGTT (17), but only one-quarter to one-half of patients with 2-h values on the OGTT ≥200 mg/dL had FPG concentrations ≥140 mg/dL (17–19). The Expert Committee decided to retain the 2-h glucose concentration of ≥200 mg/dL as a diagnostic criterion because changing it “would be very disruptive” considering the large number of epidemiological studies using that value to define diabetes.
The FPG concentration that gave a prevalence of diabetes equivalent to the 2-h value of ≥200 mg/dL on an OGTT was ∼126 mg/dL (7.0 mmol/L) (5,15,20,21) and was selected by the Expert Committee. They sought to justify the new lowered FPG criterion of ≥126 mg/dL for the diagnosis of diabetes by linking levels of glycemia with diabetic retinopathy in populations of Pima Indians (n = 960),
Egyptians (n = 1,081), and a randomly selected cohort in the Third National Health and Nutrition Examination Survey (NHANES III) (n = 2,821) FPG, 2-h OGTT glucose, and A1C levels were divided into deciles and plotted against the prevalence of retinopathy in each decile. The values reported by the Expert Committee for the first decile with an increase in retinopathy in the three studies were, respectively, as follows: FPG 136, 130, and 120 mg/dL; 2-h glucose 244, 218, and 195 mg/dL; and A1C 6.7, 6.9, and 6.2%.
These values are very misleading, however, because they were the lowest glycemic level of each initial decile in which the prevalence of retinopathy increased. Although the individual values of these patients with retinopathy were unknown, it is extremely unlikely that most of them congregated at the lower end of the decile. Using the values at the bottom of the decile for diagnosis certainly increases the sensitivity of the glucose criteria but at the usual expense of decreasing the specificity. Unfortunately, the lowest values of these deciles have been used to support the current glucose criteria for the diagnosis of diabetes.
It is much more likely that the mean/median glycemic values of the decile more truly represent the patients with retinopathy. These mid-decile values were, respectively: FPG 167, 155, and 165 mg/dL; 2-h glucose 298, 252, and 292 mg/dL; and A1C 7.8, 7.5, and 7.4%. Thus, since most people agree that the microvascular complication of retinopathy is the basis upon which glucose criteria for the diagnosis of diabetes should be chosen, the diagnosis in many individuals using the current glucose criteria are false-positives.
Further evidence that the present glucose criteria are too low if retinopathy is used to identify the glycemic levels by which to diagnose diabetes is the relationship among the microvascular complications of diabetes, glucose concentrations, and A1C levels.
Five longitudinal studies in over 2,000 diabetic patients followed from 4 to 9 years demonstrated very little development or progression of diabetic retinopathy or nephropathy if the average A1C levels were maintained between 6 and 7% and none if they were kept in the normal range below 6%. Yet, if the current glucose criteria are used, many people who are diagnosed with diabetes have normal A1C levels. For instance, in the NHANES III population with no history of diabetes, 61% and 19% of those with FPG concentrations of 126–139 mg/dL and ≥140 mg/dL, respectively, and 69% and 41% of those with 2-h glucose concentrations on an OGTT of 200–239 mg/dL and ≥240 mg/dL, respectively, had normal A1C levels. Given that bona fide diabetic retinopathy is not seen in people with normal A1C levels do we really want to diagnose diabetes in such individuals?
In contrast to the three studies allegedly supporting the current glucose criteria, three subsequent ones could not confirm threshold values for FPG or 2-h glucose concentrations on an OGTT for retinopathy. On the other hand, threshold values for A1C levels have been confirmed.
As already pointed out, there are a number of advantages to using A1C levels to diagnose diabetes, e.g., less variability of the assay compared with glucose, removal of preanalytic modifying factors, much less day-to-day variability (<2%) compared with FPG (12–15%), and better reflection of long-term glycemia. On the other hand, there are potential disadvantages, e.g., interference by hemoglobinopathies, influence of iron status and erythrocyte turnover, and increased levels in African Americans and Latinos independent of glucose concentrations. These are not insurmountable barriers.
Regarding hemoglobinopathies, in the 20 different Diabetes Control and Complications Trial (DCCT) aligned assays in use, HbS, HbC, and HbE interfere with only four and HbD with only two. In the NHANES 1999–2006 population without known diabetes, mean A1C levels were equal or 0.1% higher in iron-deficient women and men, respectively, compared with their iron-sufficient counterparts. The iron status might be evaluated in young menstruating women with A1C levels ≥6.5% before making the diagnosis of diabetes. Finally, since increased glycation is one cause of diabetes complications, the slightly higher A1C levels in minorities might have pathological significance.
In conclusion, the weakness of the evidence for the current glucose criteria to diagnose diabetes strongly supports Dr. Sacks’ contention based on measurement considerations that if A1C assays aligned with the DCCT assay are available, the diagnosis of diabetes should be made by A1C levels ≥6.5%. In addition to the measurement issues, the rationale for this conclusion is that 1) the distribution of glucose concentrations in most populations is unimodal with no consistent cut point with which to diagnose diabetes; 2) bona-fide retinopathy, a specific complication of diabetes, is not seen in people whose A1C levels are <6.5%; 3) raised A1C levels cause the microvascular complications of diabetes, and lowering levels is beneficial and increased glycation of proteins is one of the causes of diabetes complications, supplying a direct link between the diagnosis and the complications .
If a DCCT-aligned A1C assay is not available, glucose criteria can be used to diagnose diabetes. Confirmation of diagnostic values should utilize the same test to avoid confusion whereby individuals have diabetes by one criterion but not by another.
(The article is based on research by the author who is Vice Chancellor, Delhi Pharmaceutical Sciences & Research University, Delhi)