Virus, Vaccine, and Vigilance
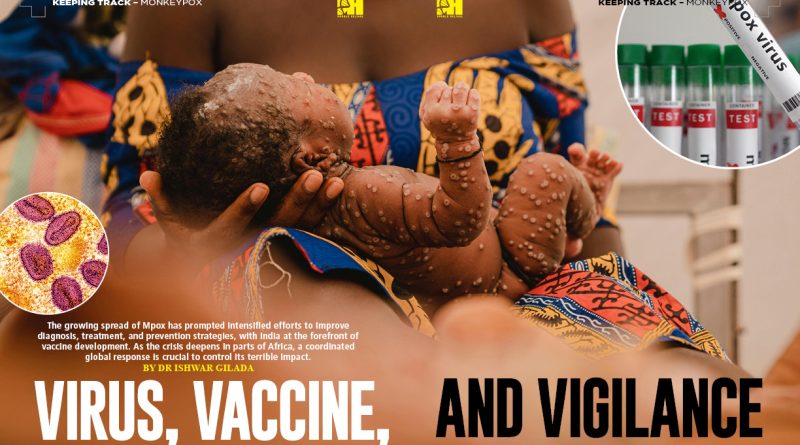
The growing spread of Mpox has prompted intensified efforts to improve diagnosis, treatment, and prevention strategies, with India at the forefront of vaccine development. As the crisis deepens in parts of Africa, a coordinated global response is crucial to control its terrible impact.
By Dr Ishwar Gilada
When the COVID-19 pandemic was receding, humanity was caught off guard by another outbreak—this time caused by the Monkeypox virus in 2022. This virus, from the Clade 2b group, spread beyond its usual regions, leading to rising cases worldwide. In response, the World Health Organization (WHO) declared Monkeypox a “Public Health Emergency of International Concern” (PHEIC) in July 2022. The emergency status was lifted in May 2023, once the situation was brought under control.
The name “Monkeypox” led to stigma and discrimination, particularly because the virus was often mistakenly associated with monkeys. Scientists and communities called for a name change to reduce this negative impact. Suggestions like “Humanpox” or “Anthropox” (meaning related to humans) were put forward. After considering different ideas, the WHO renamed it “Mpox” in November 2022 through its International Classification of Diseases (ICD-11) process.
Mpox diagnosis hinges on PCR testing, with antivirals like Tecovirimat and Brincidofovir providing treatment options, though no cure exists. The smallpox vaccine also offers significant protection.
Although smallpox was eradicated worldwide in the 1980s through vaccination, Mpox continued to appear in some African countries and occasionally outside the continent (such as a 2003 outbreak in the U.S. involving 71 cases). Between 2010 and 2022, Mpox remained endemic in 11 West and Central African countries, including the Democratic Republic of Congo (DRC), Benin, Cameroon, the Central African Republic, Sierra Leone, Gabon, Ghana, Ivory Coast, Liberia, Nigeria, and South Sudan. During the 2022-2023 outbreak, 99,176 cases and 208 deaths were reported across 116 countries, including 30 cases in India, according to WHO data.
India officially recorded its first Mpox case on July 14, 2022, in a 35-year-old man from Kerala, who had returned from the Gulf. However, the first unofficial case was in Mumbai in May 2022, involving a gay man on Pre-Exposure Prophylaxis (PrEP) for HIV prevention. He was diagnosed while working on a cargo ship but requested that his case not be reported.
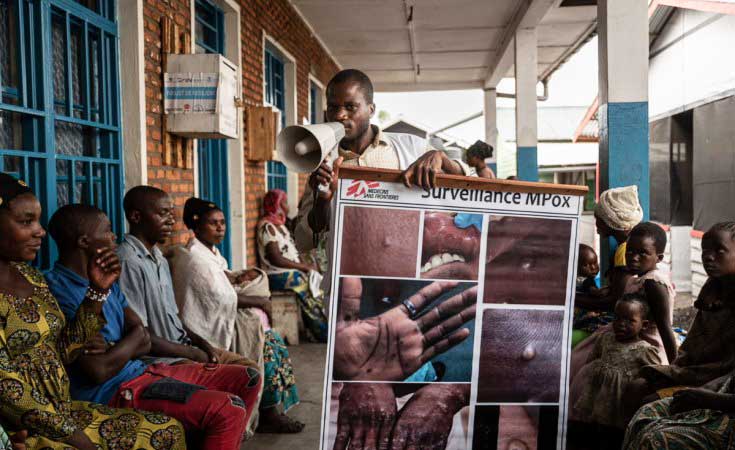
On August 14, 2024, the WHO again declared Mpox a PHEIC due to a new strain that spread from the Kivu province in the DRC to neighbouring non-endemic countries like Rwanda, Burundi, Uganda, and Kenya, and further beyond. So far, fourteen African countries and four others—Sweden, Pakistan, the Philippines, and Thailand—have reported cases. Unlike the Clade 2 strain that caused the 2022-2023 outbreak, this new strain belongs to Clade 1, more specifically Clade 1b. Clade 1b is considered more infectious than Clade 2b and has a higher mortality rate than Clade 1. Over 17,000 cases of Clade 1b have been detected in Africa, though this number is likely an underestimate due to limited resources for diagnosis and treatment.
What is Mpox?
Mpox is a viral infection caused by the Monkeypox virus (MPXV), which is considered the most significant orthopoxvirus infection since smallpox was eradicated. The orthopoxvirus group belongs to the Poxviridae family, which contains 83 species, including:
1. The Variola virus, which causes smallpox.
2. The Vaccinia virus, used in the smallpox vaccine, still seen in Brazil.
3. The Molluscum contagiosum virus, common in children and pets, and also seen in adults, especially in the genital and anal areas, as a sexually transmitted infection.
4. The Cowpox virus, found in Europe.
5. The Rabbitpox virus, discovered in the US in 1933, which does not infect humans.
6. The Ectromelia virus (ECTV), responsible for mousepox. Other members include Camelpox, Horsepox, Monkeypox, and Alaskapox, discovered in 2015.
Mpox causes symptoms similar to smallpox, though generally less severe.
Origin of the Monkeypox Virus
The Monkeypox virus was first identified in 1958 when two outbreaks of a pox-like illness occurred among laboratory monkeys in Denmark, leading to its name. The first human Mpox case was recorded in 1970 in the DRC (then known as Zaire) during a campaign to eradicate smallpox. Mpox is a zoonotic disease, meaning it spreads from animals to humans. The virus is often found in areas close to tropical rainforests, where animals such as squirrels, Gambian pouched rats, dormice, and some monkey species carry the virus. More recently, it has spread between humans. Notably, the first cases of HIV, Ebola, and Zika viruses were also traced to the DRC.
India is ramping up its vaccine production capabilities, with the Ministry of Health aiming to tackle the spread of Mpox through indigenous solutions like the MVA vaccine, following the global call for heightened prevention.
The Monkeypox Virus (MPXV): MPXV is a double-stranded DNA virus that belongs to the orthopoxvirus group within the Poxviridae family. There are two main genetic groups, or clades, of the virus:
1.The Central African (Congo Basin) Clade (Clade 1):
o Clade 1a: Found in the Congo Basin, it spreads through household contact and often affects children.
o Clade 1b: A more infectious variant that spreads beyond endemic regions, often through sexual contact. This mutated version, Clade 1b, is responsible for the current outbreak.
2. The West African Clade (Clade 2):
o Clade 2a: Found in West Africa.
o Clade 2b: This variant spread beyond endemic regions and caused the 2022-2023 Mpox outbreak.
So far, Cameroon is the only country where both clades have been found.
Schematic diagram:
Transmission: Monkeypox virus (MPXV) was initially transmitted to humans through contact with infected animals or contaminated materials. However, the current primary mode of transmission is human-to-human. The virus can enter the body via broken skin, the respiratory tract, or mucous membranes such as the eyes, nose, mouth, genitals, and anal canal. It spreads through intimate contact, respiratory droplets (not aerosols), and contaminated objects/materials. Bodily fluids, including seminal and vaginal secretions, may also play a role in transmission.
Individuals at higher risk include those who have had sexual contact with a person diagnosed with Mpox, household members of an infected person, and housekeeping personnel handling contaminated bedding without proper personal protective equipment (PPE).
Risk factors for Mpox include:
• Sexual partners of diagnosed or suspected Mpox cases, particularly within the MSM (men who have sex with men) population, which accounted for about 99% of cases during the 2022-23 outbreak.
• Close casual contact with someone diagnosed or suspected of having Mpox.
• Travel within the past three weeks to regions with reported Mpox cases (though this risk factor may be misleading).
In a study of 1,242 initial Mpox cases in 2022, 99% of the patients were male, predominantly MSM, with only 14 (1%) being women, and no children reported. Data from the European Surveillance System (TESSy) on 892 lab-confirmed Mpox cases in 2022 showed:
• Information available for 430 patients,
• 423 (98.4%) self-identified as MSM, and 99.4% of them were male,
• About 45% of patients were between the ages of 31 to 40.
Despite the predominance of sexual transmission, Mpox is not currently classified as an STI/STD. Nevertheless, the risk of transmission is not limited to MSM. Anyone in close contact with an infected person is at risk. Learning about Mpox is critical to curbing the spread, similar to the early history of HIV transmission, which initially appeared limited to MSM but later expanded to broader populations.
Despite being largely self-limiting, Mpox has emerged as a global health concern, especially with complications like secondary infections, pneumonia, and encephalitis in high-risk individuals.
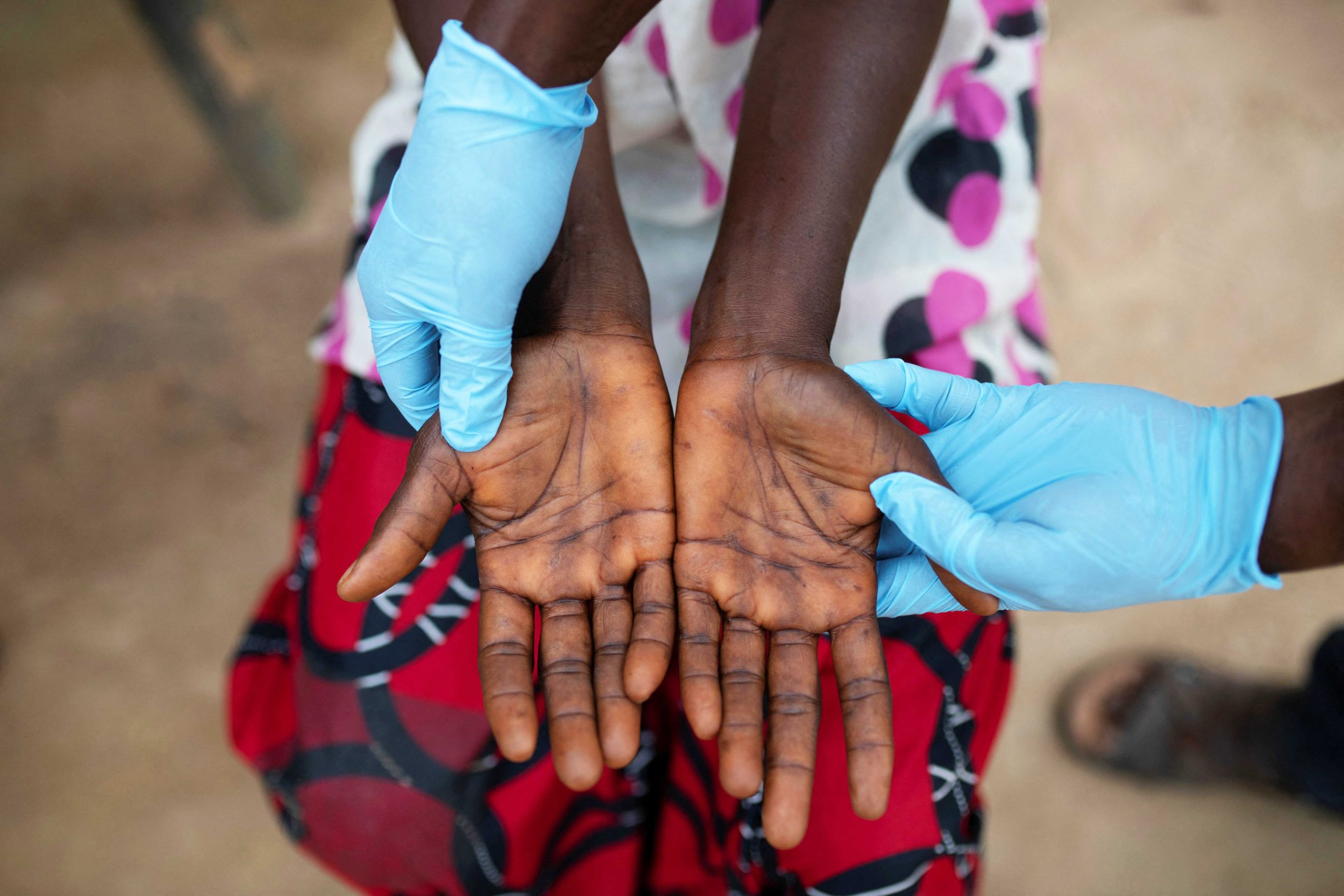
Clinical Profile: Symptoms: Mpox typically starts with fever, headache, muscle aches, back pain, and exhaustion. Lymphadenopathy (swollen lymph nodes), a feature absent in smallpox, is common. A rash resembling urticaria forms, progressing to vesicular or pustular blisters, beginning on the face and spreading to other parts of the body, including the palms, soles, and genital areas. The rash resolves through desquamation. Throughout this clinical process, patients remain infectious.
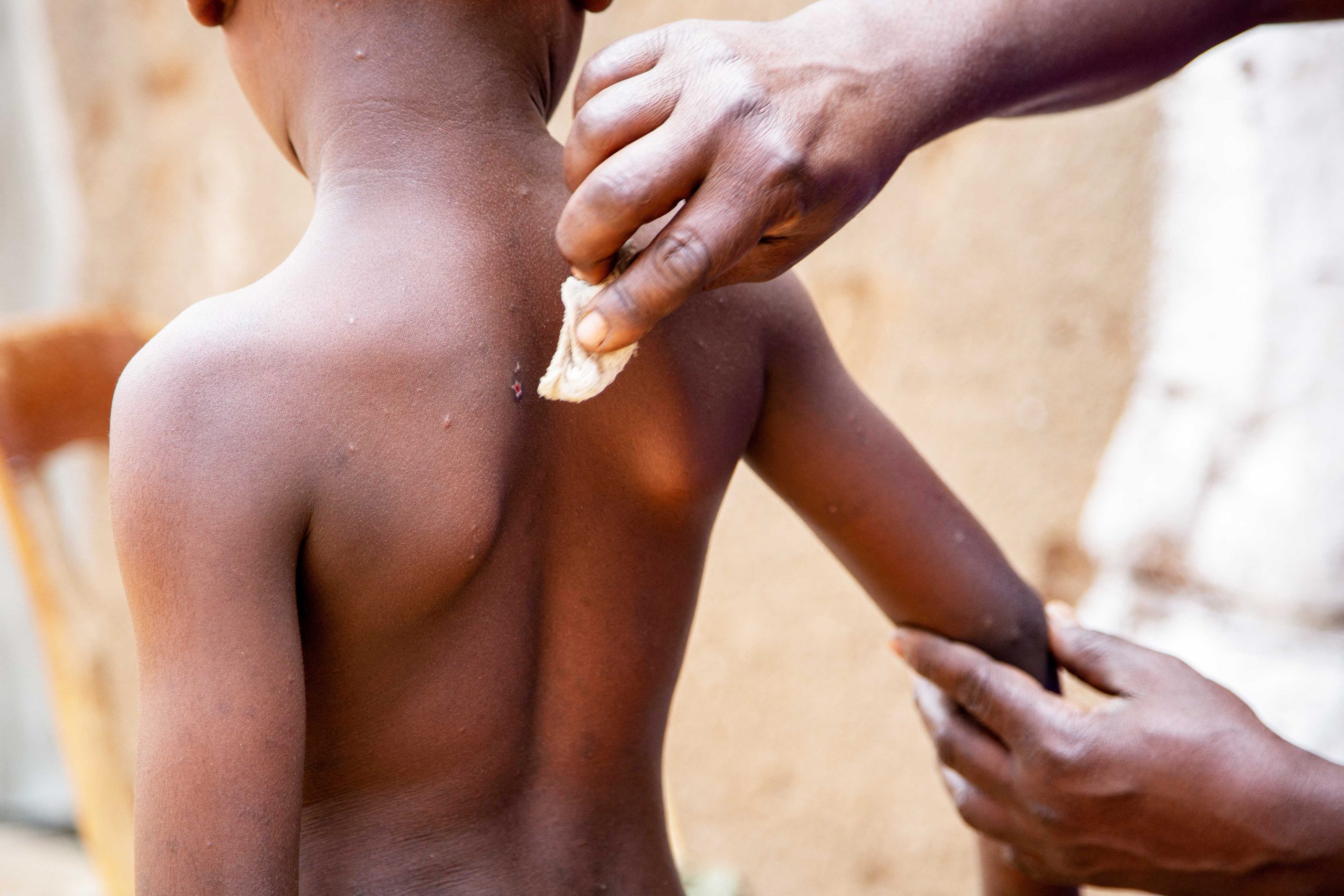
The incubation period for Mpox ranges from 5 to 21 days, and isolation of diagnosed or suspected cases is recommended for 21 days.
During the 2022 outbreak, the clinical presentation of Mpox was distinct from classical descriptions. Symptoms included only a few or a single lesion, often in the genital or peri-anal area, with limited spread. Lesions appeared asynchronously and could precede constitutional symptoms like fever.
During the 2022-23 outbreak, all patients experienced fever, rash, cervical and inguinal lymphadenopathy, and dysphagia or odynophagia. Many patients had rashes on their palms and soles, oral ulcers, myalgia, arthralgia, intense fatigue, headache, conjunctivitis, dyspnea, and anorexia. Cough, diarrhoea, and vomiting were also noted. Some patients presented with co-infections, such as Mpox with HIV or Covid-19.
Mpox must be differentiated from chickenpox, bilateral varicella zoster, measles, bacterial skin infections, scabies, syphilis, pemphigus vulgaris, bullous pemphigoid, and drug allergies.
Mpox as an STI: Mpox is emerging as a new sexually transmitted infection (STI/STD)—potentially the 21st STD of the 21st century.
Case Study: One of my PrEP patients, a 30-year-old MSM named Mr XY, was on ship duty and had unprotected sex during docking in Europe. Although PrEP protected him from HIV, he disregarded the risk of other STIs, despite counselling. Over the years, I have never encountered a case where various genital lesions appeared one after another, incubating and emerging in the peri-anal region, with solitary lesions on the face and forearm being mistaken for insect bites.
Sequentially, we suspected various infections: Herpes Simplex Virus (HSV) lesions, for which we started him on Acyclovir from the ship’s medical kit; then Molluscum Contagiosum; then secondary syphilis, and finally, Chancroid, as he had multiple painful ulcers with dirty granulation tissue. Treatment was initiated with whatever antibiotics were available on board.
What was most baffling in this case was the genital lesions, despite the patient reporting only receptive anal sex. The possibility of multiple STIs from a single episode didn’t seem convincing at first. However, upon further probing, he revealed a history of engaging in multiple sexual encounters with 8-10 partners in one night, facilitated by the use of aphrodisiacs and recreational drugs, a scenario now popularly known as ‘Chem-Sex.’ This prompted me to suspect Mpox, given his recent travel history to Europe, engagement with multiple MSM partners in one night, the evolution of lesions into vesicles/pustules on the forearm, face, peri-anal region, and genitals in a centrifugal distribution, along with associated constitutional symptoms. Moreover, three of his five known partners from that night had already been diagnosed with Mpox, and none of the treatment I had initially prescribed seemed to help him, except for painkillers.
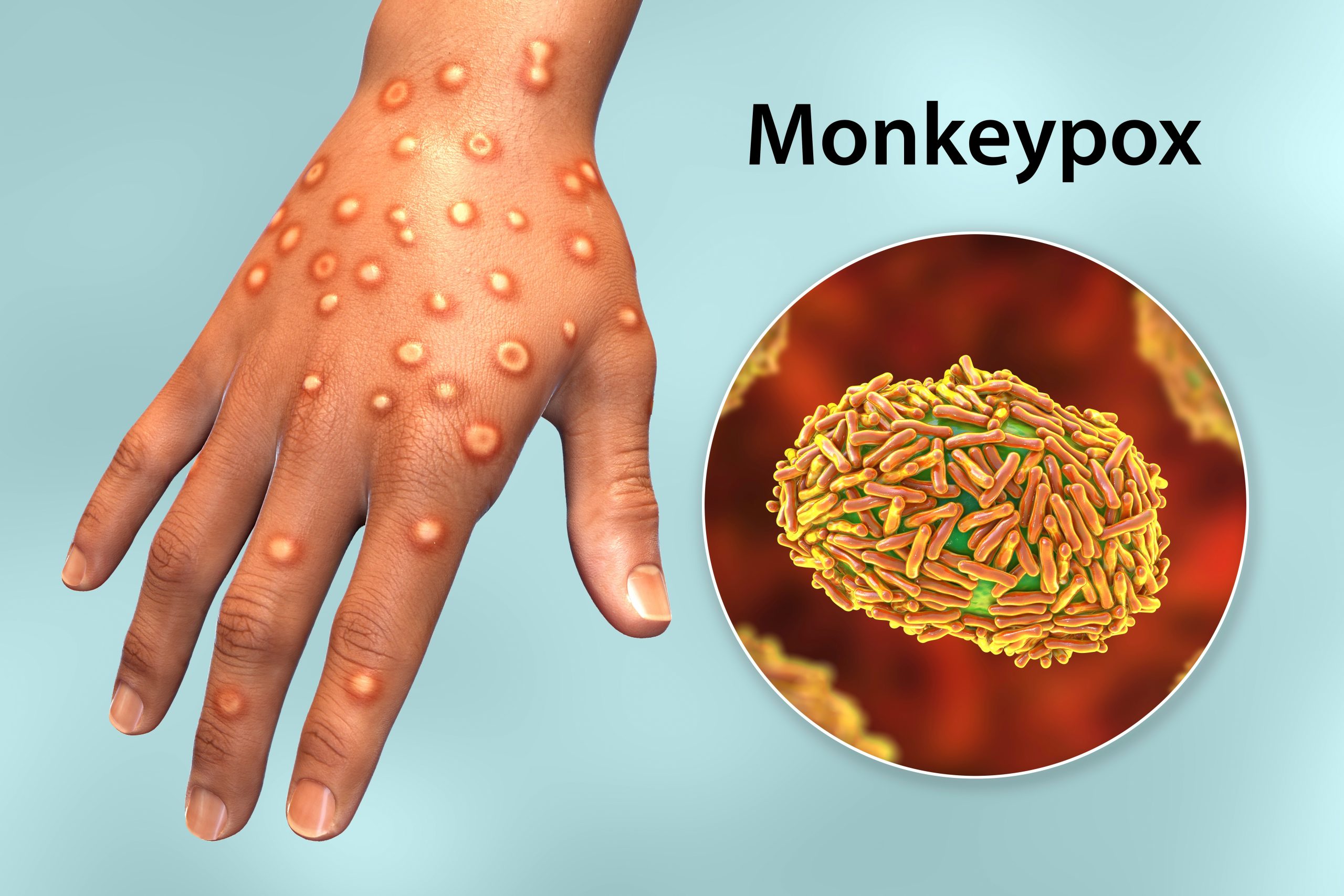
Investigations
• MPVX is detected through polymerase chain reaction (PCR) using a Nucleic Acid Amplification Test (NAAT), either real-time or conventional PCR, to detect unique sequences of viral DNA. The best diagnostic samples are taken with swabs directly from one or more vesicles, pustules, ulcers, or by dry scraping of the scabs, or through biopsy where feasible. PCR can be used alone or combined with sequencing. An indigenous PCR kit, developed in partnership with Transasia, was recently launched in Andhra Pradesh.
• Routine Biochemistry, Haematology, and Urinalysis
• Sodium and potassium (to gauge fluid loss)
• Proteins – especially albumin
• Routine examination of bacterial and fungal cultures and sensitivity
Complications and Sequelae: In most cases, Mpox is self-limiting, and patients typically recover on their own. However, the illness can be severe or lead to complications and eventual death in some people.
• Features predictive of illness severity and poor outcomes include compromised skin and mucosa.
• Systemic illness
• Hypoalbuminemia and low hematocrit, suggestive of malnutrition
• Bronchopneumonia
• Secondary infections, sepsis, septic shock with bacterial pneumonia
• Encephalitis
• Corneal infections leading to loss of vision
• Mortality: Documented cases report a mortality rate ranging from 0 to 11%, with higher rates observed among young children in endemic regions. Mortality rates can also rise due to factors like limited access to proper healthcare, comorbidities, and immunosuppression.
Treatment: Currently, there is no fully safe or proven treatment for Mpox, but the following antivirals are being used:
1. Tecovirimat (ST246) (marketed as TPOXX by SIGA, USA): This antiviral medication, used for Smallpox, Mpox, and Cowpox, interferes with the VP37 protein found on orthopoxviruses, preventing the virus from reproducing and spreading. Research on its efficacy for treating Clade 1b Mpox is ongoing.
o Dosage and Schedule: 600 mg orally, twice daily for 14 days, taken within 30 minutes of a meal. Tecovirimat is considered the first-line treatment, especially for severely immunocompromised patients. The CDC in the USA recommends Tecovirimat for children and adolescents as well.
2. Brincidofovir (CMX 001) (marketed as Tembexa by Chimerix, USA, approved in June 2021): An antiviral for CMV and HSV, it releases Cidofovir intracellularly.
o Dosage: 200 mg orally, once a week for two doses.
3. Cidofovir: Approved for medical use in 1996, it treats CMV retinitis in HIV patients.
o Dosage: 5 mg/kg body weight via IV infusion over 1 hour, administered once a week for two doses.
4.Smallpox Vaccine: If administered within 3 to 5 days of the onset of Mpox symptoms, it can help accelerate recovery.
Standard care should factor in these treatments and assess them in different settings alongside antivirals. It is also essential to focus on:
• Preventing and treating secondary infections and complications
• Ensuring adequate hydration and nutrition
• Protecting vulnerable anatomical areas such as the eyes, genitals, and peri-anal regions
Prevention of Mpox
1. Isolate infected individuals to prevent transmission to others at risk.
2. Practice good hand hygiene, including washing hands with soap and water or using alcohol-based hand sanitizers after contact with infected animals or humans.
3. Avoid direct contact with materials such as bedding or laundry that have been in contact with an Mpox patient. The virus can be eliminated through standard washing machine cycles with warm water and detergent.
4. Refrain from contact with animals that might carry MPVX, particularly in endemic areas, and avoid using creams, powders, or lotions made from wild animals.
5. Use appropriate personal protective equipment (PPE), including gowns, gloves, respirators, and eye protection when caring for patients, as per risk assessment.
Vaccine: Vaccination is a key preventive measure. The Smallpox vaccine offers excellent protection against MPVX. Cases in adults over 40 are rare, possibly due to residual immunity from smallpox vaccination campaigns before 1980. Even if an individual contracts Mpox, the clinical course tends to be mild.
• Modified Vaccinia virus Ankara (MVA): A third-generation smallpox vaccine, MVA was developed in Munich, Germany, and further manufactured by Bavarian Nordic. Marketed under the brand names Jynneos (USA), Imvamune (Canada), and Imvanex (EU and UK), this vaccine is licensed for the prevention of Mpox in those over 18 years of age and is administered subcutaneously in two doses, 28 days apart. Japan’s KM Biologics has also produced a smallpox vaccine, and Pune-based Serum Institute of India (SII) is in the process of developing and manufacturing a smallpox/Mpox vaccine.
• Aventis Pasteur Smallpox Vaccine (APSV): Available as an investigational vaccine, APSV can be manufactured under Emergency Use Authorisation (EUA) at short notice. The vaccine can reduce symptom severity, including pain from lesions, and the need for hospitalisation in Mpox patients.
• Post-exposure prophylaxis (PEP): As per the CDC, the MVA-BN vaccine is the only vaccine authorised and recommended for PEP in children and adolescents. Vaccination a few days after exposure offers adequate protection.
With African nations like the Democratic Republic of Congo facing critical shortages of Mpox vaccines and testing, global collaboration is essential to prevent the outbreak from escalating into a more severe crisis.
Takeaways
Mpox continues to spread and is expected to further propagate, requiring precautionary measures to detect cases early, isolate patients, and prevent transmission. Though there is no perfect anti-Mpox treatment, antivirals can be administered on a case-by-case basis. The primary approach remains symptomatic and supportive care, treatment of secondary infections, fluid management, and prevention of complications. The Smallpox vaccine is useful for both treatment and prevention.
India exemplifies turning adversity into opportunity, expanding its capabilities in Smallpox vaccine production. The Ministry of Health and Family Welfare, particularly NACO and ICMR, should proactively incorporate newer STDs and diseases affecting key populations into their scope of work.
The Mpox outbreak in the WHO African Region in 2024 has affected fifteen countries, as well as four countries outside of Africa. Without intervention, the trajectory of this outbreak could mimic the severity of the 2022 outbreak. The Democratic Republic of the Congo (DRC) is in acute crisis, facing a demand for 3 million Mpox vaccine doses and limited access to testing, resulting in an underestimation of cases. Global support is critical to addressing these needs. Heightened surveillance, improved preparedness, and scaled-up preventive measures in healthcare and community settings are essential for controlling the outbreak and reducing transmission. Meticulous contact tracing should suffice in managing the spread of Mpox, with no need for lockdowns or mass vaccination.
(The author is a Consultant in HIV and Infectious Diseases at Unison Medicare & Research Centre, Mumbai, Secretary General of the Organised Medicine Academic Guild, President Emeritus of the AIDS Society of India, and a Governing Council Member of the International AIDS Society.)